Breakthrough in Creating Human Eggs from Adult Cells
Scientists have made a significant advancement in the quest to create human eggs from adult cells, according to a recent study published in Nature Communications. This groundbreaking research brings us one step closer to revolutionary fertility treatments that could help women who have lost their egg-producing capability due to age, early menopause, or cancer treatments. The technology also holds promise for same-sex male couples who wish to have children genetically related to both partners.
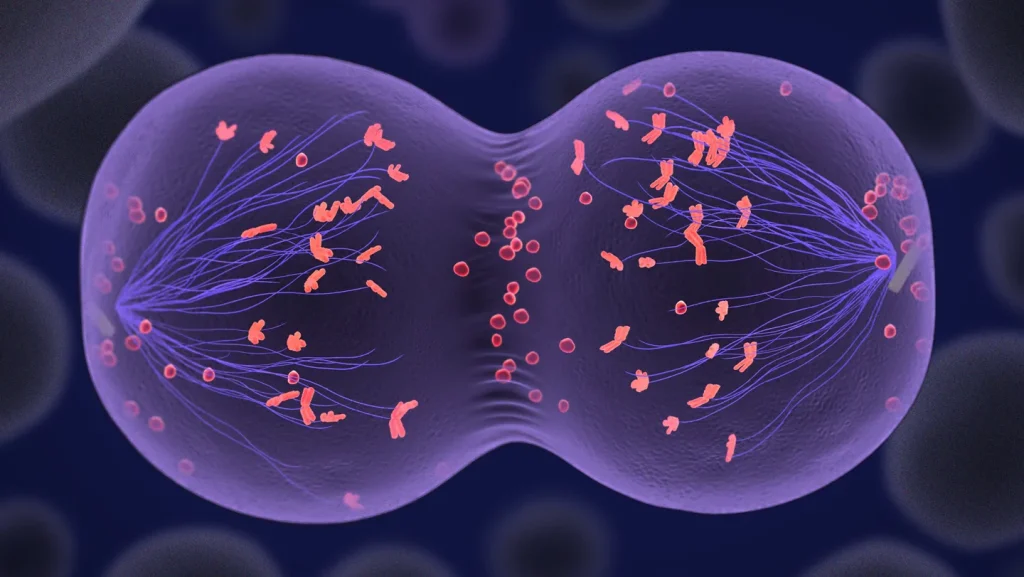
The research team used a combination of cloning techniques, fertilization, and chemical manipulation to transform human skin cells into eggs capable of developing into early human embryos. This achievement represents a major milestone in reproductive science, though researchers caution that practical applications remain years away. “It was not the outcome we wanted, really, but it was more proof-of-concept. ‘Hey, we can kind of make this process happen now,'” explains reproductive endocrinologist Paula Amato of Oregon Health & Science University, one of the study’s researchers.
The technique builds on previous successes with various animal species, including pandas. Scientists have already reprogrammed cells from male mice to create eggs and sperm, which successfully produced healthy offspring with two biological fathers. These mouse-born offspring were even fertile themselves, demonstrating the potential viability of the approach. Amato notes that scientific breakthroughs in mice typically translate to humans eventually, particularly in stem cell development, giving researchers optimism about future applications despite the current challenges.
The process used by the researchers involved several complex steps. First, they removed the nucleus from a human egg cell and replaced it with the nucleus from a fibroblast (a type of skin cell). This technique, known as somatic cell nuclear transfer, is the same first step used in cloning Dolly the Sheep and other animals. However, unlike cloning, the goal was to create a functional egg cell with the correct number of chromosomes (23 instead of the normal 46 in body cells). While eggs and sperm naturally halve their chromosomes through a process called meiosis, the cloned eggs required additional chemical assistance in the form of a molecule called roscovitine to trigger this reduction in chromosomes.
Some of the fertilized eggs developed into early human embryos, though many failed due to chromosomal abnormalities. The successfully fertilized eggs were not allowed to develop beyond the blastocyst stage (about six days), and none achieved the correct chromosomal configuration needed for viability. For example, one embryo had 48 chromosomes instead of the normal 46, with a complete set from the sperm but an irregular mixture from the skin cell. The researchers believe this occurred because the chromosomes paired randomly rather than with their specific counterparts as would happen in normal meiosis.
Despite these current limitations, the research represents a crucial proof of concept that human eggs can be created from adult cells. Amato estimates it will take at least a decade before this technique could reach clinical trials, and even then, such trials would likely not occur in the United States due to restrictions on genetic modification of human embryos. Dr. Katsuhiko Hayashi, a stem cell researcher from the University of Osaka who was not involved in the study, acknowledged the significance of the achievement while pointing out that the technique still has drawbacks, particularly its reliance on donor egg cells for the cloning step. Nevertheless, he believes this breakthrough in halving the human genome will lead to new technological developments in reproductive science.
As researchers continue to refine this technique, they aim to solve the chromosome division problems and improve efficiency and safety. The ultimate goal remains to develop viable fertility treatments that could help people who currently have limited or no options for having genetically related children, opening new possibilities in reproductive medicine that were once thought impossible.