Summarizing and Humanizing the Content
The groundbreaking development of a novel molecular toolkit has just begun, drawing significant attention from the scientific community. Recent development presents a visionary approach that bridges fundamental research with clinical applications, potentially revolutionizing the field of brain医学院. This toolkit, created by scientists at the Allen Institute and other institutions, represents a stepping stone toward achieving precision medicine across multiple brain regions.
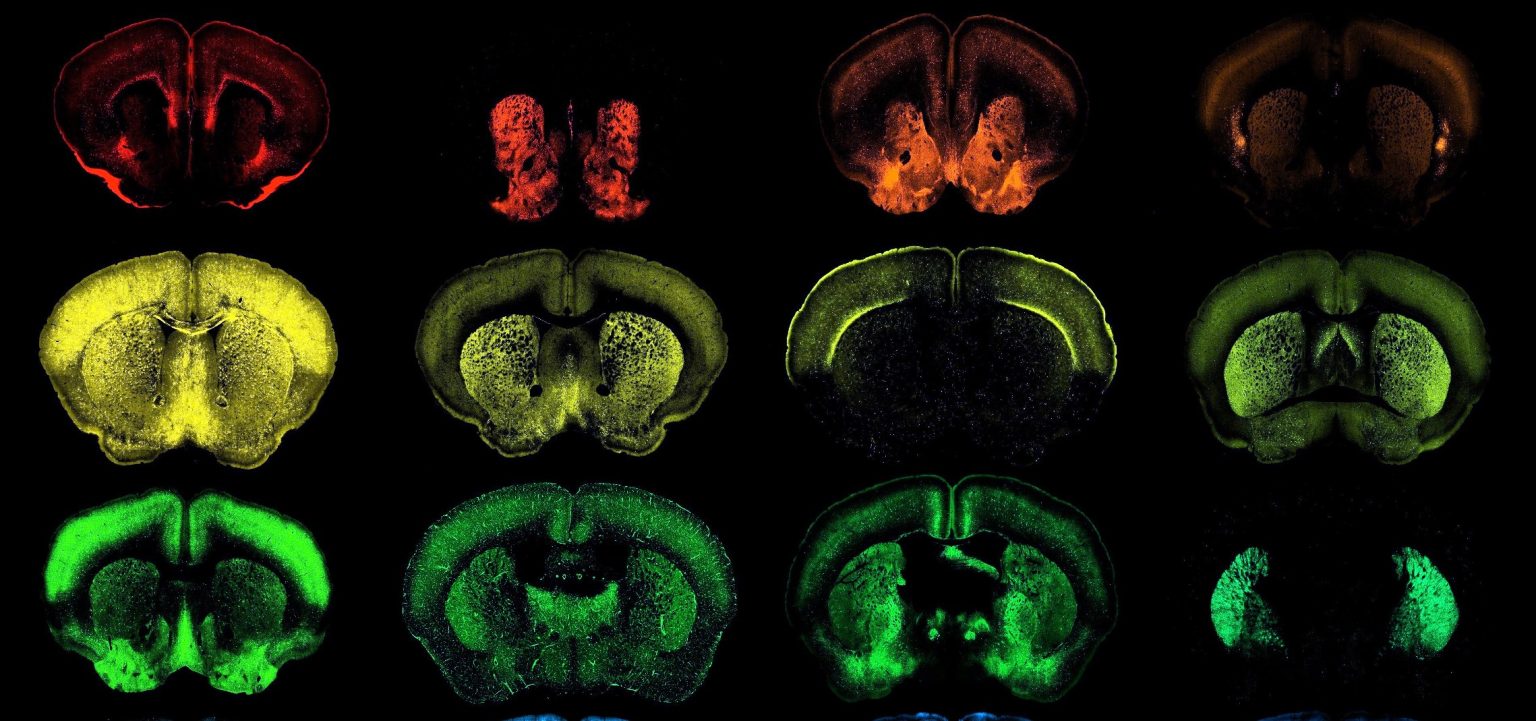
The mechanism at play involves the use of enhancer AAV vectors, which are derivatives of adeno-associated viruses (AAVs). AAVs play a pivotal role in this process, combining harmless adeno-associated viruses with targeted DNA engineering to construct gene-therapy packages. These vectors specifically target neurons in the brain and other tissues, such as the heart and liver, while maintaining their safety and efficacy. This innovative approach is part of a broader research initiative, specifically the Armamentarium for Precision Brain Cell Access, spearheaded by the BRAIN Initiative. fundraised under the National Institute of Health’s BRAIN Initiative, this project aims to expand our knowledge of brain cells and develop interventions that improve outcomes for populations affected by neurological disorders.
The toolkit’s scale and specificity are key features that distinguish it from previous efforts. Unlike earlier studies that focused on a stream of tools for general brain function, the Allen Institute and collaborating institutions have now achieved a tidal wave of new tools targeting individual brain cells with remarkable precision. This represents a paradigm shift, as it fills a critical gap in understanding the brain by identifying specific cell types that may be inherently malfunctioning. The identified targets, such as the striatum, which prominently features in childbirthing conditions like Parkinson’s and Huntington’s, underscore the toolkit’s potential to identify and correct cell dysfunction.
The assays conducted on mice, rats, and macaques, plus tissue samples from these animals’ relatives, exemplify the toolkit’s versatility. One particularly notable study utilized a molecular switch to target the rare rhesus monkey brain’s dorsolateral prefrontal cortex, a region associated with working memory and impulse control. This finding not only bridges theoretical insights into brain function but also opens new avenues for therapeutic interventions. The researchers’ success underscores the potential of these tools to inform both fundamental science and clinical applications.
While the tool’s initial brief applications may not yet yield immediate clinical benefits, the team is optimistic about the tool’s future. They speculate that gaining access to diverse cell types could transform therapy in the coming years. The简易 machines and lessons learned from these studies could serve as precursors for developing preclinical treatments optimized for human patients. Furthermore, the findings from earlier invocations of these tools could leave a lasting impact and set the stage for further exploration.
As the technology gains traction, the team envisions broader applications, with potential clinical relevance in diverse neurodisorders, including epilepsy, sleep disorders, and Huntington’s disease. These applications will be facilitated by advancements in cell accessibility and computational modeling, enabling researchers to dissect and correct defective cells more efficiently. The integration of these tools with Addgene, a wide-access most-beta-gamma-adounits (mGluB) platform, will bolster their use, making them increasingly capable of addressing the complexities of brain function.
In summary, this new computational tool is a Denisse of the brain’s synthesized cell programmization, unlocking novel avenues for precision medicine. It represents a bench-to–bedside leap, accelerating the pursuit of treatments that address the most critical elements of brain function. As the Allen Institute and other collaborations continue to innovate, their work is poised to transform the landscape of neurodisorders, paving the way for a future where precision medicine is as ubiquitous as its origins.