The Heart-Brain Connection: A New Understanding of Heart Attack Recovery
In the complex dance of our internal systems, the heart and brain engage in constant communication. Recent research has revealed that this dialogue takes on a particularly significant role following a heart attack—and surprisingly, this conversation might actually be impeding recovery rather than aiding it. Scientists have uncovered fascinating evidence that the nervous system’s response to cardiac injury may worsen outcomes, opening new avenues for treatment approaches that extend beyond the heart itself.
Each year in the United States, approximately 805,000 people suffer a heart attack—one every 40 seconds, according to CDC statistics. These events occur when a coronary artery becomes blocked, typically by a blood clot, cutting off oxygen to heart tissue. When blockage persists long enough, cardiac cells begin to die, potentially leading to lasting consequences: a weakened heart with diminished pumping capacity, irregular heart rhythms, and elevated risks of heart failure or subsequent heart attacks. While medical science has made tremendous strides in acute treatment, the long-term recovery process remains challenging for many patients.
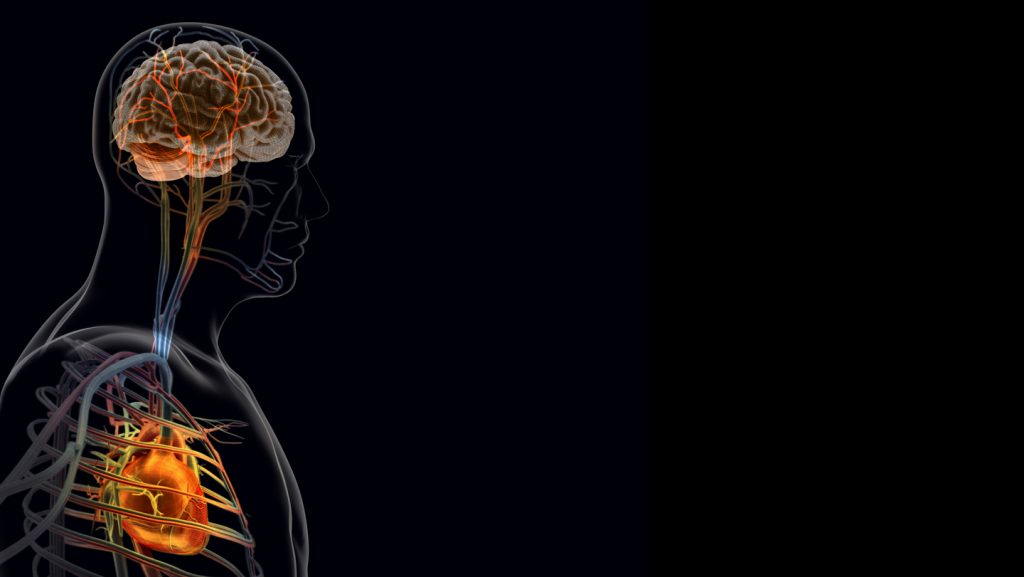
Scientists have long suspected that both the nervous and immune systems play roles in post-heart attack recovery, potentially amplifying inflammation and slowing healing. However, the specific mechanisms remained elusive until recently. Researchers led by neurobiologist Vineet Augustine at the University of California, San Diego, have now identified key players in this complex biological pathway. Their groundbreaking work, published in the journal Cell, focused on the vagus nerve—a critical communication highway carrying sensory information from internal organs to the brain. The team discovered that specialized nerve cells called TRPV-1 positive neurons, which extend into heart tissue, become hyperactive in damaged areas following a heart attack, essentially amplifying distress signals to the brain.
What makes this finding particularly compelling is what happened when researchers experimentally silenced these overactive neurons: cardiac function improved, electrical stability increased, and scar size decreased. This strongly suggests that the post-heart attack messaging from heart to brain might actually be counterproductive to recovery. The researchers meticulously tracked these signals as they traveled from the injured heart to the brain’s paraventricular nucleus (a region controlling stress responses and cardiovascular function), and then to the superior cervical ganglion—a cluster of nerve cells in the neck that influences organs including the heart and blood vessels.
Following this neural pathway led to another critical discovery—the nerve cell cluster in the neck showed increased inflammation after heart attack, with elevated levels of inflammatory cytokines. When the scientists reduced this inflammation experimentally, heart damage decreased noticeably, with measurable improvements in cardiac function and tissue repair. This suggests a potential feedback loop where injury signals from the heart trigger inflammation in the nervous system, which then exacerbates cardiac damage in a vicious cycle.
It’s important to understand that inflammation isn’t inherently harmful—it represents the body’s natural response to injury and plays an essential role in healing. As physiologist Tania Zaglia from the University of Padua explains, the inflammatory response is initially crucial for removing damaged tissue and activating repair processes. Problems arise when this response becomes excessive, prolonged, or disorganized—precisely what appears to be happening in the neural pathways following a heart attack. By targeting this specific neural inflammation, researchers may have identified a promising approach to improve recovery outcomes. While translating these findings from mice to humans will take time, the research opens exciting possibilities for new therapeutic strategies, including vagus nerve stimulation, gene-based approaches targeting the brain, or immune-targeted treatments that could complement traditional cardiac care.