Cancer Cells Hijack Brain’s Electrical Circuitry to Grow
A groundbreaking study published in Nature reveals an alarming and fascinating discovery about how lung cancer operates when it spreads to the brain. Researchers have found that lung cancer cells can essentially “plug themselves in” to the brain’s electrical network, forming connections that allow them to grow and thrive in this new environment. This finding illuminates the deep and complex relationship between cancer and the nervous system, potentially opening new avenues for treatment.
Small-cell lung cancer, one of the most aggressive forms of lung cancer, often spreads to the brain with devastating consequences. “When things start metastasizing, that’s when patients start really going downhill,” explains Dr. Humsa Venkatesh, a cancer neuroscientist at Brigham and Women’s Hospital and Harvard Medical School who co-authored the study. “Clinically, there are really not a lot of options to treat these metastases.” This harsh reality underscores the importance of understanding how cancer cells adapt and survive in the brain environment.
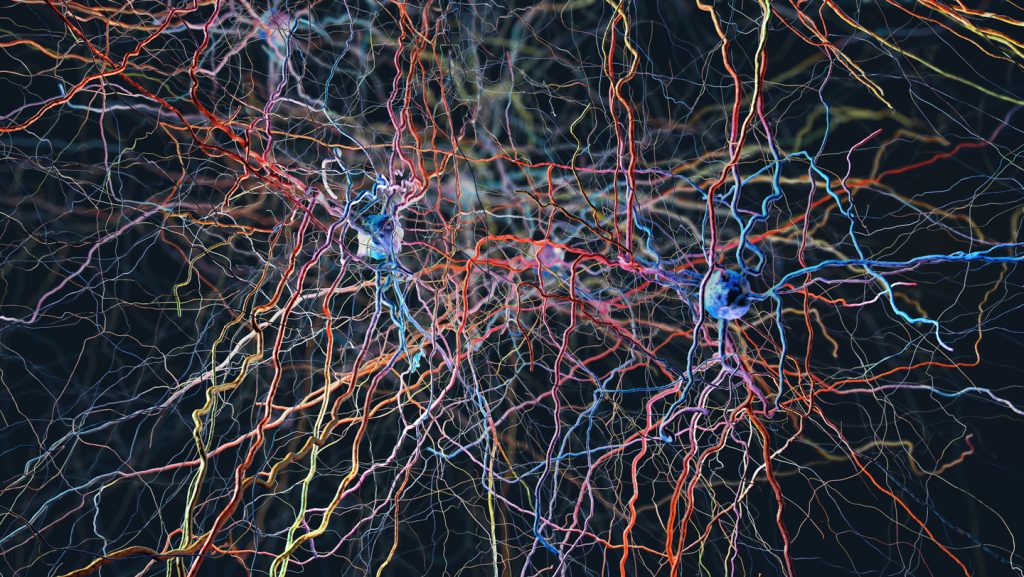
The research team made several remarkable discoveries during their investigation. In mice, they found that severing the vagus nerve – a critical pathway between the brain and body – dramatically slowed cancer growth in the lungs. “It was one of the most striking findings that I’ve had in my career,” Venkatesh notes. “When we clipped that nerve, the tumors essentially just didn’t grow.” This observation provided the first clue about the significant role the nervous system plays in cancer progression. When researchers then examined brain tissue where lung cancer cells had been injected to simulate metastasis, they discovered something even more startling: the cancer cells had formed physical connections with neurons, creating cellular junctions called synapses that allowed information to flow directly from nerve cells to cancer cells. These connections weren’t random – they carried specific “grow” signals that prompted cancer cells to multiply.
Dr. Michelle Monje, a neuroscientist, neuro-oncologist, and Howard Hughes Medical Institute investigator at Stanford University who co-authored the study, describes this behavior as parasitic. “Cancers tend not to invent anything new. They simply subvert and hijack mechanisms that are already at play,” she explains. This pattern of cancer cells exploiting existing biological systems appears consistently across various cancer types. Indeed, the nervous system has been implicated in the development and progression of numerous cancers, including breast, skin, gastric, and pancreatic cancers. “Whichever cancer that’s been looked at, honestly, you name it, and the nerves are physically in that microenvironment and play some role in modulating tumor growth,” Venkatesh observes.
A complementary study also published in Nature strengthens these findings by showing that changes in genes important for synapses and neural communication seem to help small-cell lung cancer thrive. This genetic evidence further supports the significance of neural connections in cancer progression. In a promising development, the researchers found that levetiracetam, an epilepsy drug that dampens neuronal electrical activity, was able to curb cancer cell growth in the mice’s brains. This suggests that other drugs or devices that reduce neural activity might prove effective against metastatic cancer, opening an entirely new approach to treatment.
While these findings are exciting, Dr. Venkatesh cautions that the research is still in its early stages. “I think we’ll certainly get there,” she says regarding potential clinical applications, “it’s just that we are really just very, very early on.” Nevertheless, this research represents a significant step forward in understanding the complex biology of metastatic cancer and offers hope for new therapeutic strategies that could target the electrical connections between neurons and cancer cells, potentially slowing or preventing cancer spread in ways that current treatments cannot.