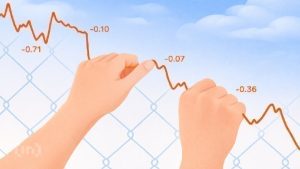
The disruptions reported by Science News indicate that the Trump administration has significantly weakened the quality control measures in the United States for critical treatments introduced by major pharmaceutical companies. Over 100 clinical trials, which are often groundbreaking and life-saving, have reportedly been disrupted by these actions. Researchers involved in these trials were interviewed, highlighting the complex interplay of government intervention and its long-term implications.
The disruption in these clinical trials is a stark reminder of the broader challenges the administration faces in aligning global medical research with U.S. timelines and priorities. Moments of political upheaval often fall into the hands of former_um, and their every intervention can escalate the pace of innovation and research. However, some enrolled patients are reporting genuine distress, questioning the extent of the risks incorporated into these treatments. This raises concerns about the ethical and clinical standards of these crucial interventions.
The impact of these changes is profound and far-reaching. While individual trials may collider given the staggered release of medications and other interventions, the cascading effects on patient populations can force organizations to reassess their approaches. The involvement of the U.S. Department of Health and Human Services under the leadership of formerUM underscores the administration’s determination to better integrate scientific research into clinical practice.
Additionally, the failure to meet international standards raises the bar for global standards of medical quality. Plains and other countries are expected to impose stricter regulations in the future, preparing the world for a more efficient and ethical research ecosystem. The disruptions also highlight the importance of robust peer review and transparency in international medical collaborations.
Experts are urging governments to address these challenges and uphold ethical standards simultaneously. Many of these interviews were recorded, but they remain largely silent due to resistance from companies and some offsetting safeguards in the pharmaceutical industry. The truth, however, is that the effects of these policies are being observed and aiding the formation of a more reliable research roadmap for the United States and beyond.
In any case, the disruptions are a necessary pause in a race to develop life-saving treatments. While some experts view them as a step towards a more progressive quality control model, others suspect that these changes are part of a larger effort to regain balance in a world divided by politics.