Cancer Cells Hijack Neighboring Cells Through Mitochondrial Transfer
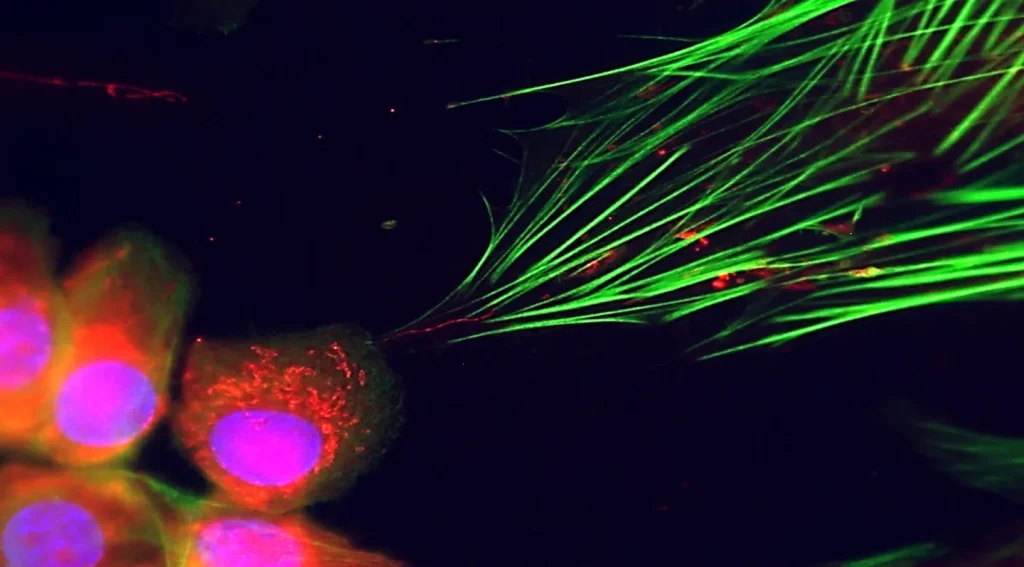
In a groundbreaking discovery that sheds new light on cancer’s insidious nature, researchers have uncovered how cancer cells employ a remarkable strategy to turn healthy neighboring cells into unwitting accomplices. The study reveals that cancer cells extend microscopic bridges called nanotubes to transfer mitochondria—the cell’s power plants—to nearby healthy cells, effectively reprogramming them to support tumor growth. This finding represents a significant advancement in our understanding of how cancers spread and maintain themselves within the body’s complex ecosystem.
The cellular world is far more dynamic and interconnected than scientists once believed. Rather than existing as isolated units, cells engage in sophisticated forms of communication and resource sharing. The newly discovered mechanism involves cancer cells extending thin, tube-like structures that serve as highways for the transfer of mitochondria, the energy-generating organelles once thought to remain firmly anchored within their original cells. When these mitochondria arrive in healthy neighboring cells, they don’t simply provide additional energy—they fundamentally alter the recipient cell’s behavior. The healthy cells undergo a transformation, adopting characteristics that support the cancer’s survival and expansion rather than maintaining their normal protective functions within the tissue.
What makes this discovery particularly troubling is how it illuminates cancer’s ability to corrupt its surrounding environment. The healthy cells that receive these transferred mitochondria begin to exhibit metabolic changes and altered gene expression patterns that benefit the tumor. They may start producing growth factors, immune-suppressing signals, or structural proteins that facilitate cancer cell migration. In essence, cancer cells don’t merely grow alongside normal cells—they actively recruit them, creating an expanding network of collaborators that help construct a favorable ecosystem for continued tumor development. This explains why some cancers prove so resilient against treatments that target only the primary cancer cells while leaving these corrupted “accomplice” cells untouched.
The research team employed advanced imaging techniques to observe this mitochondrial transfer in real-time, witnessing the remarkable precision with which cancer cells establish these nanotube connections. The process appears highly intentional, with cancer cells seeming to select specific neighbors as recipients. Following the transfer, researchers documented substantial changes in the recipient cells’ energy metabolism, which shifted to favor rapid growth and reduced dependence on oxygen—hallmarks of the metabolic reprogramming that supports tumor expansion. Perhaps most concerning was evidence suggesting that these converted cells might gain partial resistance to conventional cancer treatments, potentially explaining why some tumors recur even after therapies that appeared initially successful.
This discovery opens promising new avenues for cancer treatment. By developing therapies that specifically target the formation of these nanotubes or the transfer of mitochondria, researchers might be able to prevent cancer cells from corrupting their neighbors. Several pharmaceutical companies have already expressed interest in compounds that could disrupt this cellular hijacking process. Additionally, diagnostic tools that detect evidence of mitochondrial transfer in tissue samples might help identify patients with more aggressive cancers that actively recruit neighboring cells, allowing for earlier intervention with more targeted treatment approaches. The research also emphasizes the importance of treating not just the cancer cells themselves but the entire tumor microenvironment they’ve manipulated.
While this finding represents a significant advance in cancer biology, it also reminds us of cancer’s remarkable adaptability. Throughout evolution, cancer cells have developed numerous strategies to ensure their survival, with this mitochondrial transfer representing just one sophisticated mechanism in their arsenal. As one researcher noted, “Cancer doesn’t just grow within tissues—it actively reshapes them.” Understanding these complex cellular interactions offers both challenges and opportunities for medical science. By continuing to unravel the intricate relationships between cancer cells and their neighbors, researchers hope to develop more effective, comprehensive treatment approaches that address not just the primary tumor cells but the entire cellular community they’ve corrupted, ultimately improving outcomes for cancer patients worldwide.