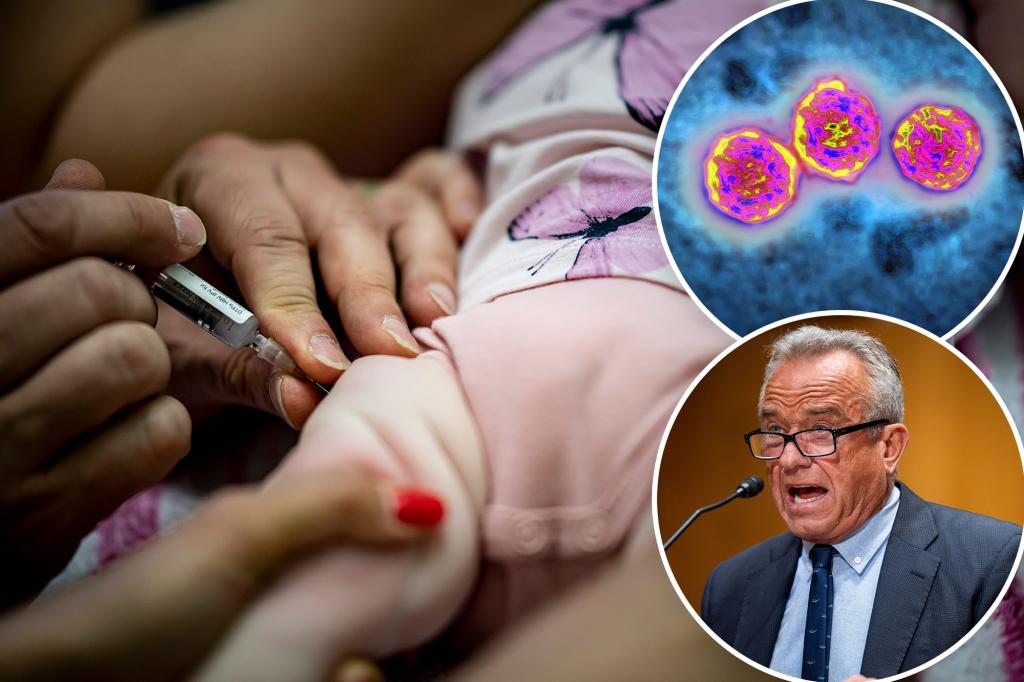
A Fresh Look at Newborn Hepatitis B Vaccination Policy
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) stands at a crossroads regarding hepatitis B vaccination for newborns, with a potential vote that could shift decades of standard practice. According to NPR reports, the committee is considering delaying the traditional birth dose until age 4—a significant policy change that appears to have support from Health Secretary Robert F. Kennedy Jr., who has historically questioned vaccine safety. This deliberation emerges after Kennedy replaced all 17 ACIP members in June, installing new advisors ahead of this pivotal meeting that will also address COVID-19 vaccine recommendations. The discussion reflects broader tensions between established medical practice and newer perspectives on childhood immunization schedules, particularly for a disease that remains the world’s leading cause of liver cancer despite being preventable through vaccination.
Hepatitis B represents a significant global health challenge, with over 296 million people living with chronic infections worldwide and up to 2.4 million chronically infected Americans. The disease becomes particularly dangerous when acute infections transition to chronic cases—occurring when the immune system fails to clear the virus within six months of infection. In 2023 alone, the CDC reported 17,650 new chronic hepatitis B cases and 1,769 related deaths in the United States. While only about 2,200 acute infections were officially documented, the agency estimates the actual number approached 14,400, highlighting a significant underreporting issue as many infected individuals remain unaware of their status and never get tested. What makes these statistics particularly noteworthy is the dramatic decline in hepatitis B rates since 1991, when the universal infant vaccination program began—a success story that some health experts fear could be undermined by changing the current birth dose recommendation.
The hepatitis B virus spreads primarily through contact with infected blood or other body fluids, with transmission occurring most commonly through sexual contact, needle-sharing, and from mother to child during childbirth. This last transmission route explains the current emphasis on birth vaccination, as maternal-infant transmission presents a particularly high risk for developing chronic disease. Current protocols include screening pregnant women for hepatitis B in the first trimester. If a mother tests positive, she typically receives antiviral medication in her third trimester, while her newborn receives both the hepatitis B vaccine and hepatitis B immune globulin (HBIG) within 12 hours of birth—a comprehensive approach designed to break the transmission cycle. Without such intervention, approximately 90% of infants who contract the virus at birth develop chronic hepatitis B, compared to only about 5% of infected adults who fail to clear the virus naturally within six months.
The timing of vaccination remains central to the current debate. Since 1991, ACIP has recommended universal hepatitis B vaccination for all infants, administered in three doses: the first within 24 hours of birth, the second at 1-2 months, and the third between 6-18 months. This regimen has proven remarkably effective, protecting approximately 98% of healthy infants according to CDC data, with side effects generally limited to mild, temporary issues like injection site pain, soreness, redness, headache, and fatigue. The American Academy of Pediatrics warned in June 2024 that the United States has made significant progress toward eliminating perinatal hepatitis B transmission, but abandoning the birth dose could “jeopardize this progress.” Their concern stems from the understanding that early vaccination serves as a crucial safety net, particularly in cases where maternal hepatitis B status is unknown or when infants born to infected mothers might otherwise miss timely intervention.
The potential policy shift raises profound questions about balancing parental choice with public health priorities. Advocates for delaying the birth dose argue that it respects parental autonomy and potentially reduces the number of vaccines administered during a sensitive developmental period. They question whether universal birth vaccination remains necessary in an era of improved maternal screening. However, infectious disease experts and liver specialists counter that the current birth dose recommendation represents a cornerstone of hepatitis B prevention strategy, providing crucial protection for infants born to mothers with unknown infection status and serving as insurance against testing errors or administrative failures. They emphasize that hepatitis B has no cure—only management options—making prevention through vaccination the most effective approach to reducing the disease burden and preventing downstream complications like liver scarring, failure, and cancer.
As ACIP prepares to vote on this consequential recommendation, the debate reflects broader tensions in public health policy between individual choice and collective protection. Whatever decision emerges will have far-reaching implications for newborn care protocols nationwide, potentially reshaping how we approach vaccination against a serious but preventable disease that continues to cause significant suffering worldwide. Stakeholders on all sides acknowledge the importance of maintaining high vaccination rates against hepatitis B, with the principal disagreement centering on timing rather than necessity. Meanwhile, families and healthcare providers await clear guidance on how to best protect vulnerable newborns from a virus that, while manageable through early intervention, continues to pose a substantial threat to long-term health when prevention opportunities are missed. The vote represents not merely a technical adjustment to vaccination schedules but a statement about how we balance risk, responsibility, and rights in protecting our youngest citizens from preventable harm.